Haloalkanes 7. Elimination to form an Alkene.
One in a series looking at the chemistry of haloalkanes (alkyl halides).
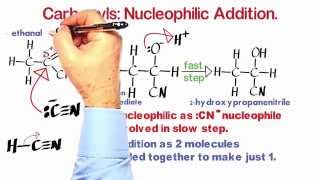
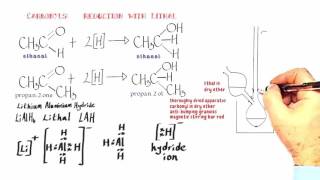
This video looks at the reaction of bromoethane with an ethanolic solution of potassium hydroxide. The contrast is drawn between nucleophilic substitution and elimination...
One in a series looking at the chemistry of haloalkanes (alkyl halides).
This video looks at the reaction of bromoethane with an ethanolic solution of potassium hydroxide. The contrast is drawn between nucleophilic substitution and elimination mechanisms. In substitution the hydroxide ions behave as nucleophiles, whereas in elimination, the hydroxide ions act as a Bronsted base bringing about deprotonation.
More...
Collapse
23 Views
Comments (0)
Please log in to post comments.