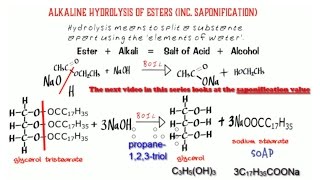
Haloalkanes 5. Rate of hydrolysis shown & explained.
This video both demonstrates and explains the factors that control the rate of hydrolysis of haloalkanes. 1-chlorobutane should be more reactive than 1-iodobutane, on the basis that the C-Cl bond is more polar than the C-I...
This video both demonstrates and explains the factors that control the rate of hydrolysis of haloalkanes. 1-chlorobutane should be more reactive than 1-iodobutane, on the basis that the C-Cl bond is more polar than the C-I bond - thus attracting the nucleophile better. However, 1-iodobutane would be expected to react faster on the basis that the C-I bond is weaker than the C-Cl bond, thus lowering the activation energy of the reaction. This video identifies which of the two factors has the greater influence on the rate.
More...
Collapse
20 Views
Comments (0)
Please log in to post comments.